Archives
29/06: Curious about Water? Read on....

IN its very deceptive ORDINARINESS, water is exceptionally extraordinary. It is almost everywhere - in air; clouds; oceans; lakes; rivers; springs or glaciers. In the five-kilometer layer below the sea level on Earth, water is nearly six times as abundant as all other substances put together. Not only has it been the cause of rise and fall of great civilizations, it has also been one of the agents responsible for shaping and reshaping the face of Earth. Falling as rain or flowing in rivers, it levels mighty mountains, creates broad valleys or steep canyons by weathering the hardest rocks. Availability of water determines the economy of an agrarian society like India. As steam or hydroelectric power, it drives the wheels of modern technology, it's an indispensable ingredient in nearly all manufacturing processes, from the baking of breads to cutting steel for automobiles. Last but not the least it is the elixir of life. Life has evolved in water, and is designed around it, many organisms can live without air, but none without water. Obviously water arouses very many questions in a fertile young mind. Here are the answers to a few such questions.
How did water originate on Earth?
- Why does water around toothpaste dropped on a wet floor drain away?
- Why does a drop of oil expand over surface of water while a drop of water over the surface of oil contracts to become round shaped?
- Why water cannot be used for frying like oil?
- Why are organic compounds not soluble in water?
- Why don't oil and water mix together?
- Why is it that we can blow bubbles only with a soap solution?
- Why do impurities settle down on addition of alum to dirty water?
- Why does water get cold on addition of glucose to it?
The origin of water on Earth is linked to the formation of Earth. According to some currently accepted theories Earth began as a waterless mass of rock surrounded by cloud of gas. Radioactive materials in the rock and increasing pressure in the Earth's interior gradually produced enough heat to melt the interior of the Earth. The heavy materials, such as iron ores, then sank. The light silicates (rocks made up of silicon and oxygen) rose to the Earth's surface and formed the earliest crust. Many silicate rocks have water molecules integrated into their atomic arrangement --water can be driven out of such rocks by the action of heat. Thus the heating of the Earth's interior caused release of water contained in such rocks to the surface. Over millions of years, water thus released collected slowly in low places of the crust and formed the oceans. Whatsoever might have been the origin of water, Earth's original supply of water is still in use and very little, if any, has been added during the past billion years or so. The same water has been pumped time and again from the oceans into air, dropped upon the land and transferred back to sea. A single drop of water spends 8 to 10 days passing through air, 2 to 3 weeks in a river, as long as 100 years in a Himalayan glacier, or from 100 to 40,000 years underground.
As a chemical substance water is unique and rather odd. All its oddities can be traced to its molecular structure. It is a rather sturdy molecule. Until some 180 years ago water was believed to be an indivisible element rather than a chemical compound. Today any student of science knows that each of its molecule is made up of two atoms of hydrogen and one atom of oxygen. The bond between the oxygen and the hydrogen atom in water is polar, that is, it has positive and negative charged ends because of an unequal distribution of electrons. The oxygen atom has a denser distribution of electrons around it and hence a net negative charge. The hydrogen atoms in a water molecule, on the other hand, are positively charged. This leads to a lopsided molecule, with electrical charges concentrated on opposite sides. Water molecules, are therefore, attracted to each other as well as to other molecules having a similar charge distribution. And many of the characteristic features of water can be traced to the so-called hydrogen bond between its molecules.
When a substance dissolves in another substance, the resulting distribution of the molecules of the two substances has lesser number of molecules of either substance surrounded by its own kind. This necessitates disrupting prevailing intermolecular forces in each of them. The molecules of most organic compounds (e.g. oily substances) are non polar. As a consequence the intermolecular forces between organic molecules are much weaker than in water. If such a substance is to mix with water -the resulting distribution of molecules must lead to lowering of energy content. If more energy is required to separate water molecules from each other (by breaking hydrogen bonds) than is gained when water molecules get closer to the organic molecules, the two substances will not mix together. It is for this reason that water and oil do not mix and many organic compounds do not dissolve in water. Oil spreads on the surface of water for a similar reason, because in doing so the interaction of water molecules with the oil molecules is minimized. However, not all organic compounds are insoluble in water. Glucose is a common example. It is an organic compound, which dissolves in water. This is because the molecule of glucose has several hydrogen--oxygen bonds, which can indeed interact with the hydrogen-oxygen bonds of water favorably. In the process of mixing glucose molecules with water some energy is consumed. This energy is acquired from the heat energy of the water, hence water becomes cooler when glucose is dissolved in it.
When a foodstuff is fried some water is removed from it since the cooking medium is at a temperature higher than the boiling point of water. Oils have a boiling point much higher than that of water, hence frying is defined as a process of cooking in excess of fat or oil, thus cooking in water cannot be called frying.
Molecules of some of the compounds used to make soap and tooth paste have a long non polar chain like molecules, but on one end of these chains there is a polar bond. Dissolved in water, they form a structure in which all the non polar chains are stacked together in a layer, so that they have a minimal contact with water molecules. When some toothpaste is dropped on a wet floor some of its molecules spread out on the nearby area, and since they are hydrophobic (they repel water) water layer recedes from this area. When we blow bubbles using a solution of soap in water soap molecules rearrange from a micellar (spherical globules) to a lamellar (like a thin film) arrangement such that air can be trapped in between. Molecules of water themselves cannot arrange themselves into such stable arrangements, hence we cannot blow bubbles using pure water because while a soap solution can form a stretchable film plain water cannot.
When alum (aluminum sulfate) is added to dirty water, a gelatinous precipitate of aluminum hydroxide (known as flocs) is formed. When this precipitate settles down it carries with it bacteria, mud and other impurities as they stick to it, thus clearing the turbidity.
Rivers, Ponds, Wells and Oceans
- Why is the seawater salty?
- From where do the rivers like Godavari and Kaveri get water since they are not formed from glaciers like the river Ganga and Yamuna?
- Why does sea water makes soil infertile whereas river water doesn't?
- Why are there no tides in a pond, like in the oceans?
- Why does the water from a well or tubewell feel warm in winter but cold in summer?
- On islands in the sea, from where do the people get drinking water?
- When bubbles are at the bottom of a pond or river they are small but they get bigger as they come to the surface?
- Although water in ponds, etc. remains fresh for years, kept in a vessel why does it often get contaminated after a few days?
- How can we perform a test at home to check if the water from a handpump is suitable for drinking?
- How does the seawater move?

As rivers flow over land they dissolve many minerals on the way to the oceans. Oceans and seas therefore contain the washings of the entire land area of Earth of several million years. Many undersea volcanic activities as well as underground springs also add salt to the sea. Most of these salts remain entrapped in sea water, and thus sea water is salty. Rivers originate either on mountain glaciers or underground springs at high altitudes. River water therefore has very low salt content and does not taste salty.
Most plants cannot withstand high salinity of the soil because the water uptake by a plant through its roots takes place due to osmosis. If the salt content of the water in the soil is higher than in the plant cells water will tend to flow out of plant tissue rather into it-resulting in its drying up. Soils irrigated with sea water are therefore infertile for common plants, however, there are plants like mangroves which can survive in soils having high salinity.
Rivers like Ganga originate from the glaciers on Himalayas hence they are perennial. Peninsular rivers like Godavri, Krishna or Narmada, on the other hand, are largely rain fed. They have their origins in western ghats (or Vindhyachal). Rain water accumulated underground in such mountainous ranges comes out in the form of numerous springs which merge together to form these peninsular rivers.
The periodic rise and fall of the oceans, or other large bodies of water, relative to the surrounding land is commonly known as tides. They are produced by a combination of number of external forces, with the principal force being the gravitational attraction of the Moon. If the Moon attracted every point within the Earth with equal force, there would be no tide. It is the small difference in direction and magnitude of the lunar attractive force, on one point of the Earth's mass relative to another, which give rise to the so-called tidal stresses. Oceans and seas are spread over a much larger area than any pond or lake. The maximum possible difference in gravitational attraction due to Moon within a pond (or lake) is therefore much smaller than in oceans and seas. Thus although a pond or a lake is subject to the same forces the oceans, the observed changes in water level in them is much smaller. For example, Lake Superior, a very large lake between the United States and Canada, has tides that rise and fall about 5 centimeters compared to the rise and fall of a few meters in some oceans.
There are currents in the oceans which make water move in them. Water in an ocean moves because of drifts of water due to temperature differences or the prevailing winds above. Thus ocean currents are fast flowing currents of seawater generated by the wind or by the variations in water density between two areas.
The specific heat of water, that is, the heat energy required to raise the temperature of 1 gram of water by 1 degree Celsius is relatively high. Thus temperature of a large mass of water on the Earth changes rather sluggishly. Water in a well is largely insulated from the temperature variations in the atmosphere above as it has only a very limited contact with it. The temperature of groundwater, therefore, does not vary much through various seasons. During winters it feels warmer because the atmospheric temperature is relatively lower and during summers it feels cold because the atmospheric temperature is relatively higher.
Drinking water on oceanic islands is usually obtained from rain-fed lakes or groundwater which is sweet.. Normally groundwater (which can be pumped up) is fit for human consumption, because as the water percolates into an underground reservoir most of the microbes get filtered off. However, if the soil contains substances from which harmful ions can leach out, the water which passes through it may become contaminated. There have been reports of arsenic leaching into underground water in certain areas in West Bengal. Testing such water often requires chemicals and apparatus not readily available at home.
The quantity of water in a pond is much more than in any domestic vessel. It is ultraviolet radiation from the Sun and the flora and fauna inside a lake or a pond, which keeps it clean. Water from such a pond, if kept in a domestic container, may contain spores of some bacteria, which may grow in it, thus contaminating it. However chlorinated water kept even for several months in a domestic pot does not get infected because the dissolved chlorine prevents any microbial growth.
The external pressure of water on air contained in a bubble deep down in a pond is much more than the atmospheric pressure. Thus as an air bubble rises it is subject to lesser and lesser pressure. It therefore grows bigger. In addition small bubbles coalescing together as they rise also give rise to larger bubbles.

Ice and Snow
- Why do pipes carrying water often burst in cold countries during winter?
- A shower of hail often accompanies the first rain after summer season, why?
- Why are the hailstones hard while the snowballs soft?
- Whereas common salt is often added to the frozen snow in Kashmir during the winters -- to melt it, during summers in Delhi ice-cream hawkers add common salt to ice-jackets surrounding ice-cream container so that ice will not melt. Why this contradiction?
- Why is snow softer than ice?
- Why does ice melt but waxes harden when subjected to pressure?
- Why does water occupy less volume than the ice formed by same amount of water?
- Why does the level of water in a container remain unchanged when an ice cube floating in it melts?
- Why do small pieces of ice stick together to form a block when pressed?
- Can water be converted to ice by application of pressure?
- Why are ice cubes preferred to water at zero degree Celsius to cool a soft drink?
- Why does ice melt on addition of salt?
- Why is there no snowfall in deserts although hailstone showers as precipitation are common?
When water is cooled to 0 degree Celsius it freezes, that is, it crystallizes into ice, it expands. This is so because the structure of ice is such that the water molecules are spaced farther apart than in liquid water. Water molecules therefore occupy less space as liquid water than as ice and the density of liquid water is more than that of ice. Ice therefore floats on water and when floating ice melts it does not result in any increase in volume. It is for this expansion of water that water pipes if kept closed at the ends often burst in winter if the ambient temperature drops below zero.
Hail consists of rounded particles of ice which fall out of cumulonimbus clouds. This class of clouds are characterized by a tall mountain like appearance and they span from about 1000m. to 7000m. in the atmosphere above the sea level. Such clouds are formed by a strong vertical movement of hot humid air, which is possible mainly in deserts or plains near them where there has been a spell of hot summer. No wonder thunderstorms accompanied by hail are common only in such regions. Larger hailstones commonly exhibit an onion like structure of alternating shells of clear and opaque ice. The clear ice layer consists of large crystals of water that has cooled rather slowly. The thinner opaque layer contains air bubbles and is often an accumulation of snow crystals.
Snow is a conglomerate of many small ice crystals. A snowfall can take place only in such places where the ambient temperature falls below zero degree Celsius for a rather long spell of time. As the packing of such crystals can change with the application of pressure snow is softer than ice or hailstones. When a block of ice is subject to external pressure it melts because the crystal structure of water molecules in ice breaks down under the pressure and water molecules cannot be crystallized in an alternate crystal structure unless the pressure is drastically raised above 2000 atm. However, as soon as the pressure is released the water at zero degree Celsius freezes back into ice. Thus when we press together two different pieces of ice, it melts at the boundaries due to increased pressure and then freezes back on release of pressure. The ice thus formed at the periphery is common to both pieces hence the two pieces join together into one piece. Water can be converted into ice only by application of phenomenally very high pressures. At a pressure of about 20,000 atm, liquid water freezes to ice VII at about 1000C.
Ice is preferred over water at zero degrees Celsius to cool a drink because ice ( at 00C) can absorb much more heat (than water at 00C) before it is converted to water at say 10 degrees Celsius. This is so because water at 00C loses some more heat (known as latent heat of fusion ) before it is converted to ice at 00C.
The transition temperature of pure water into ice is zero degree Celsius at normal atmospheric pressure. But this transition point shifts to a lower temperature ( about -150C) if sufficient salt is added to water. A mixture of ice and salt at a temperature above its fusion temperature will therefore be converted to a liquid salt-water solution by absorbing heat from the surroundings. Thus when salt is added to snow on roads covered with snow in Srinagar, the snow melts. Ice cream vendors in Delhi add a lot of salt to ice in the ice cream trolleys (almost in the ratio 1:1). In the process the temperature of the mixture falls down because the process of dissolution of salt into water is endothermic, that is, it entails some absorption of heat from the surroundings. Therefore salt added to water lowers the temperature of the mixture. Some ice does melt but the undissolved salt at subzero temperature helps in keeping the ice-cream frozen for a longer period.

Vapours, Steam and Clouds
- Why does the water in a covered pot boil faster?
- We know that clouds contain +ve and -ve charges but when clouds become rain where do these charges go ?
- During natural evaporation of water, neither boiling point of water is reached nor latent heat is given. So how does water change its state?
- Clouds contain positive charges on the upper side and negative charges on the lower side(towards ground). Why the negative charge is on the lower side?
- When we pour chilled water in a tumbler, how do water droplets form on its outer surface?
- Can we save fuel by boiling water in a pressure cooker?
- Why does a direct contact with hot steam cause more severe burns than contact with boiling water?
- How is it that water can be boiled in a paper cup without burning the cup?
Water can exist in the three states -- solid, liquid and gas, simultaneously. A piece of ice at zero degree Celsius can has around it liquid water and water vapours at the same time, even though ice is the most stable state of water at this temperature. This is so because there are always some molecules in ice or liquid water which have enough kinetic energy to escape from the bulk. Thus water vapours co-exit with liquid water or ice at all temperatures. Their relative quantities depend on how far off the conditions are from the freezing or boiling point of water. Water in a pond is therefore constantly evaporating from it and condensing into it what ever be its temperature. Water on the outside surface of a cold tumbler is largely due to condensation of water in the atmosphere. Liquid water is not converted into water vapours only at 100 degrees Celsius alone. In fact if we decrease the pressure on its surface it can boil at lower temperatures.
When water boils the liquid and the vapour states are nearly equally probable. Hence if its temperature is maintained at the boiling point more and more vapours will be formed. If the vapours are allowed to escape the pressure does not rise but if they are not allowed to escape the pressure may rise and lesser heat is required to keep it boiling. In a pressure cooker water boils at a temperature higher than 1000C. because the pressure due to vapours inside it can rise much above the atmospheric pressure. It saves fuel required for cooking because heat used to vaporize water inside it does not escape with the vapours, as in an open vessel during the time taken for cooking. If we boil water in a pressure cooker, more heat will be required to raise its temperature to the boiling temperature (which will be higher than 1000C) but lesser heat may escape along with the steam as the temperature rises to the boiling point. One cannot therefore give an offhand answer because the relative masses of the container/pressure cooker also need to be considered.
One calorie of heat is enough to raise the temperature of 1 g of water at 990Ct to 1000C, the boiling point of water. But after we supply this much heat 1 g of water will not be converted to 1 g of steam. Some additional heat is required to be put in to convert water at 1000C to steam at 1000C. This additional quantity of heat is known as latent heat of vaporization. Water has a rather high latent heat of vaporization, 540 cal./g. Thus steam at 100 degrees Celsius has more heat in it than liquid water at the same temperature. No wonder steam can cause more severe burns than boiling water. Just like freezing, addition of salts to water affects its boiling also - it raises the boiling point. Hard water therefore boils at a temperature higher than 100 degrees Celsius.
When we boil water in a paper cup almost all the heat supplied from the heater is conducted off to raise the temperature of water or convert it into steam. The temperature of the paper cup remains around 100 degrees, which is not sufficient to burn it.
Cosmic rays ionize the water and air molecules in the upper strata of the clouds by knocking off electrons leading to positively charged molecules. Some of the electrons thus liberated combine with other molecules in the lower strata to give rise to negatively charged molecules. Since the Earth carries a net positive charge, these ions distribute themselves so that negatively charges ions are concentrated in the lower segment of the clouds. When the clouds precipitate as rain the negative and positive ions can interact to neutralize the charges, or the excess negative charge may flow into the Earth.

Floating and Swimming
- Why does a ship travel faster in warm water than in cold water?
- How do huge and heavy ships made of steel float while a steel spoon sinks in water?
- Why does a poorie or pakoda tends to float up to the top when it is being fried in boiling oil?
- A needle can float on the surface of water but not on the surface of a soap solution, why?
- Why is it easier to swim in a sea than in a river?
- Why do tea leaves float on top when soaked in a tea pot but sink down when stirred?
- Is there any place in the sea where the density of water is so much that a drowning ship is suspended freely instead of going to the sea bottom?
- Some people claim that certain sadhus can walk on water, is it plausible?
- Why does ice float in water but sink in alcohol?
A solid body as it sinks displaces some of the water. The water exerts an upward thrust on it. This upward thrust is equal to the weight of water the solid displaces. A ship is a hollow structure of steel since it displaces much more weight of water than its weight it floats while a spoon does not because as it sinks the weight of water displaced by it is less than its weight. The buoyancy due to different types of water depends on its density also(Archimedes principle. Seawater is denser than river water because of the dissolved salts, therefore the buoyancy in seawater is greater and lesser effort is required to swim in it. Similarly the much lower density of alcohol is responsible for insufficient thrust to keep an ice cube floating in it. However a ship travels faster in warm water than in cold water because warm water offers lesser frictional resistance to the motion of ship than cold water because of a looser packing of molecules.
The molecules on the surface of liquid water are asymmetrically attracted towards other molecules in it because there are very few molecules above the surface. This leads the surface to behave almost like a stretched membrane. A force, known as surface tension, characterizes the membrane like feature. The magnitude of surface tension is proportional to the intermolecular attraction between the water molecules on the surface. A needle can be floated on the surface of water because of the surface tension of water. When soap is dissolved in water the surface tension of water is decreased and is not sufficient to support the weight of a normal needle.
Tea leaves float in water because they have air trapped in them, when they are stirred the air escapes or gets dissolved in water. Poories and pakoras float in oil while being fried because the water vapours released (or entrapped between layers) during the process of frying make them less dense. A human being cannot walk on water because the weight of an erect human being is spread over a very small area. Thus the surface tension of water cannot hold it. Thus according to the principles of physics it is impossible that a human being can walk on the surface of water.
22/06: More and More Fun Facts....

1. Mosquito repellents don't repel. They hide you. The spray blocks the mosquito's sensors so they don't know you're there.
2. Dentists have recommended that a toothbrush be kept at least 6 feet away from a toilet to avoid airborne particles resulting from the flush.
3. The liquid inside young coconuts can be used as substitute for blood plasma.
4. No piece of paper can be folded in half more than 7 times.
5. Donkeys kill more people annually than plane crashes.
6. You burn more calories sleeping than you do watching television.
7. Oak trees do not produce acorns until they are fifty years of age or older.
8. The first product to have a bar code was Wrigley's gum.

9. The king of hearts is the only king without a mustache.
10. A Boeing 747s wingspan is longer than the Wright brother's first flight.
11. American Airlines saved $40,000 in 1987 by eliminating 1 olive from each salad served in first-class.
12. Venus is the only planet that rotates clockwise.

13. Apples, not caffeine, are more efficient at waking you up in the morning.
14. The plastic things on the end of shoelaces are called aglets.
15. Most dust particles in your house are made from dead skin.

16. The first owner of the Marlboro Company died of lung cancer.
17. Michael Jordan makes more money from Nike annually than all of the Nike factory workers in Malaysia combined.
18. Marilyn Monroe had six toes. (rumor)
19. All US Presidents have worn glasses. Some just didn't like being seen wearing them in public.
20. Walt Disney was afraid of mice.
21. Pearls melt in vinegar.
22. Thirty-five percent of the people who use personal ads for dating are already married.
23. The three most valuable brand names on earth: Marlboro, Coca-Cola, and Budweiser, in that order.

24. It is possible to lead a cow upstairs...but not downstairs.
25. A duck's quack doesn't echo and no one knows why. (Or does it? http://www.acoustics.salford.ac.uk/acoustics_world/duck/duck.htm)
26. The reason firehouses have circular stairways is from the days when the engines were pulled by horses. The horses were stabled on the ground floor and figured out how to walk up straight staircases.
27. Richard Millhouse Nixon was the first US president whose name contains all the letters from the word 'criminal.' The second was William Jefferson Clinton.
28. Turtles can breathe through their butts.
29. Butterflies taste with their feet.
30. In 10 minutes, a hurricane releases more energy than all of the world's nuclear weapons combined.
31. On average, 100 people choke to death on ball-point pens every year.
32. On average people fear spiders more than they do death.
33. Ninety percent of New York City cabbies are recently arrived immigrants.
34. Elephants are the only animals that can't jump.
35. Only one person in two billion will live to be 116 or older.
36. Women blink nearly twice as much as men.
37. It's physically impossible for you to lick your elbow. (or can you? http://www.uvm.edu/~dfisher1/random/elbow.jpg http://www.uvm.edu/~dfisher1/random/elbow2.jpg)
38. The Main Library at Indiana University sinks over an inch every year because when it was built, engineers failed to take into account the weight of all the books that would occupy the building.
39. A snail can sleep for three years.
40. No word in the English language rhymes with 'MONTH.'
41. Average life span of a major league baseball: 7 pitches.
42. Our eyes are always the same size from birth, but our nose and ears never stop growing. SCARY!!!
43. The electric chair was invented by a dentist.
44. All polar bears are left handed.
45. In ancient Egypt, priests plucked EVERY hair from their bodies, including their eyebrows and eyelashes.
46. An ostrich's eye is bigger than its brain.
47. TYPEWRITER is the longest word that can be made using the letters only on one row of the keyboard.
48. 'Go', is the shortest complete sentence in the English language.
49. If Barbie were life-size, her measurements would be 39-23-33. She would stand seven feet, two inches tall. Barbie's full name is Barbara Millicent Roberts.
50. A crocodile cannot stick its tongue out.
51. The cigarette lighter was invented before the match.
52. Almost everyone who reads this will try to lick their elbow.
22/06: Bouncy Ball and other animations
 Bouncy Ball |
Happy Hippo |
The Computer Dummies |
| Sad Life |
Me and You | Happy Happy! |
Toaster Action... | The Flower | Computers! |
21/06: The Mayonnaise Jar and 2 Cups of Coffee

When things in your life seem almost too much to handle...
When 24 Hours in a day is not enough...
Remember the mayonnaise jar and 2 cups of coffee.
A professor stood before his philosophy class and had some items in front of him. When the class began, wordlessly, he picked up a very large, empty mayonnaise jar and proceeded to fill it with golf balls.
He then asked the students if the jar was full. They agreed that it was. The professor then picked up a box of pebbles and poured them into the jar. He shook the jar lightly. The pebbles rolled into the open areas between the golf balls. He then asked the students again if the jar was full.. They agreed it was.
The professor then picked up a box of sand and poured it into the jar. Of course, the sand filled up everything else. He asked, once more, if the jar was full. The students responded with an unanimous "yes."
The professor then produced two cups of coffee from under the table and poured the entire contents into the jar, effectively filling all the empty spaces among the sand. The students laughed. "Now," said the professor, as the laughter subsided, "I want you to recognize that this jar represents your life. The golf balls are the important things - God, family hildren, health, friends, and favorite passions -- things that, if everything else was lost and only they remained, your life would still be full.
The pebbles are the other things that matter, such as your job, house, and car.
The sand is everything else --the small stuff. "If you put the sand into the jar first," he continued, "there is no room for the pebbles or the golf balls. The same goes for life.
If you spend all your time and energy on the small stuff, you will never have room for the things that are important to you.
So...
Pay attention to the things that are critical to your happiness.
Play with your children. Take time to get medical checkups. Take your partner out to dinner. Play another 18. There will always be time to clean the house and fix the disposal.
"Take care of the golf balls first --the things that really matter.
Set your priorities. The rest is just sand." One of the students raised her hand and inquired what the coffee represented.
The professor smiled. "I'm glad you asked". It just goes to show you that no matter how full your life may seem, there's always room for a couple of cups of coffee with a friend."
20/06: Interesting Facts to consider...

1. If you yelled for 8 years, 7 months and 6 days,
you would have produced enough sound energy to heat one cup of coffee.
(Hardly seems worth it)
2. If you fart consistently for 6 years and 9 months,
enough gas is produced to create the energy of an atomic bomb.
(Now that's more like it)

3. A pig's orgasm lasts for 30 minutes.
(In my next life I want to be a pig...How'd they figure this out, and why?)
4. Banging your head against a wall uses 150 calories an hour.
(Still can't get over that pig thing... Don't try this at home...maybe at work?)

5. Humans and dolphins are the only species that have sex for pleasure.
(Is that why Flipper was always smiling? And pigs get 30-minute orgasms? Doesn't seem fair)
6. The strongest muscle in the body is the tongue.
(Hmmmmmmmmm........)
7. Right-handed people live, on average,
nine years longer than left-handed people do.
(If you're ambidextrous do you split the difference?)

8. The ant can lift 50 times its own weight,
can pull 30 times its own weight and
always falls over on its right side when intoxicated.
(From drinking little bottles of...? Did taxpayers pay for this research??)
9. Polar bears are left handed.
(Who knew....? Who cares? How'd they find out, did they ask them?)
10. The catfish has over 27,000 taste buds.
(What can be so tasty on the bottom of the pond?)

11. The flea can jump 350 times its body length.
It's like a human jumping the length of a football field.
(30 minutes...can you imagine?? And why pigs?)
12. A cockroach will live nine days without it's head,
before it starves to death.
(Creepy)
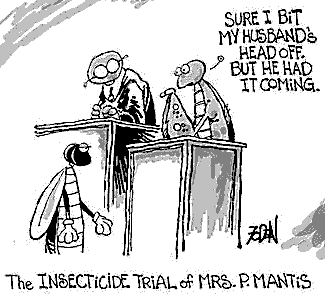
13. The male praying mantis cannot copulate while its head
is attached to its body. The female initiates sex by ripping the male's head off.
(Honey, I'm home. What the....Well, at least pigs get a break there...)

14. Some lions mate over 50 times a day.
(In my next life I still want to be a pig ... quality over quantity)
15. Butterflies taste with their feet.
(Oh, Geez... That's almost as bad as catfish)
16. An ostrich's eye is bigger than it's brain.
(I know some people like that.)

17. Starfish don't have brains.
(I know some people like that too.)
13/06: 100 Ideas by Keri Smith...
If you're bored and have nothing to do, Keri Smith (www.kerismith.com) has some artsy ideas you can try. I stumbled on her website and wanted to share her list of her 100 ideas.
1. Go for a walk. Draw or list things you find on the the sidewalk.
2. Write a letter to yourself in the future.
3. Buy something inexpensive as a symbol for your need to create, (new pen, a tea cup, journal). Use it everyday.
4. Draw your dinner.
5. Find a piece of poetry you respond to. Rewrite it and glue it into your journal.
6. Glue an envelope into your journal. For one week collect items you find on the street.
7. Expose yourself to a new artist, (go to a gallery, or in a book.) Write about what moves you about it.
8. Find a photo of a person you do not know. Write a brief bio about them.
9. Spend a day drawing only red things.
10. Draw your bike.
11. Make a list of everything you buy in the next week.
12. Make a map of everywhere you went in one day.
13. Draw a map of the creases on your hand, (knuckles, palm)
14. Trace your footsteps with chalk.
15. Record an overheard conversation.
16. Trace the path of the moon in relation to where you live.
17. Go to a paint store. Collect 'chips' of all your favorite colors.
18. Draw your favorite tree.
19. Take 15 minutes to eat an orange.
20. Write a haiku.
21. Hang upside down for five minutes.
22. Hang found objects from tree branches.
23. Make a puppet.
24. Create an outdoor room from things you find in nature.
25. Read a book in one day.
26. Illustrate your grocery list.
27. Read a story out loud to a friend.
28. Write a letter to someone you admire.
29. Study the face of someone you do not like.
30. Make a meal based on a color theme. (i.e. all white).
31. Creat a museum of very small things.
32. List the smells in your neighborhood.
33. List 100 uses for a tin can.
34. Fill an entire page in your jounral with small circles. Color them in.
35. Give away something you love.
36. Choose an object, draw the side you can't see.
37. List all of the places you've ever lived.
38. Describe your favourite room in detail.
39. Write about your relationship with your washing machine.
40. Draw all of the things in your purse/bag.
41. Make a mini book based on the theme, "my grocery list".
42. Create a character based on someone you know. Write a list of personality traits.
43. Recall your favorite childhood game.
44. Put postcards of art pieces/painting on the inside of your kitchen cupboard doors, so you can see them everyday (but not become deaf to them.)
45. Draw the same object every day for a week.
46. Write in your journal using a different medium (brush & ink, charcoal, old typewriter, crayons, fat markers.
47. Draw the individual items of your favorite outfit.
48. Make a useful item using only paper & tape.
49. Research a celebration or ritual from another culture.
50. Do a temporary art installation using a pad of post it notes & a pen.
51. Draw a map of your favorite sitting spots in your town/city. (photocopy it and give it to someone you like.)
52. Record all of the sounds you hear in the course of one hours.
53. Using a grid, collect various textures from magazine and play them off of each other. 54. Cut out all media for one day. Write about the effects.
55. Make pencil rubbings of six different surfaces.
56. Draw your garbage.
57. Do a morning collage.
58. List your ten most important things, (not including animals or people.)
59. List ten things you would like to do every day.
60. Glue a photo of yourself as a child into your journal.
61. Trasform some garbage.
62. Write an entry in your journal in really LARGE letters.
63. Collect some 'flat' things in nature (leaves, flowers). Glue or tape them into your journal. 64. Physically alter a page. (i.e. cut a hole, pour tea on it, burn it, fold it, etc.)
65. Find several color combinations you respond to in public. Document them using swatches, write where you found them.
66. Write a journal entry describing something "secret". Cut it up into several pieces and glue them back in scrambled.
67. Record descriptions or definitions of subjects or words you are interested in, found in encyclopedias or dictionaries.
68. Draw the outline of an object without looking at the page. (contour drawing).
69. What were you thinking just now? write it down.
70. Do nothing.
71. Write a list of ten things you could to do. Do the last thing on the list.
72. Create an image using dots.
73. Do 3 drawings at different speeds.
74. Put a small object in your left pocket (or in a bag), Put your left hand in the pocket. Draw it by feel.
75. Create a graph documenting or measuring something in your life.
76. Draw the sun.
77. Create instructions for a simple everyday task.
78. Make prints using food. (fruit and vegetables cut in half, fish, etc.)
79. Find a photo. Alter it by drawing over it.
80. Write a letter using an unconventional medium.
81. Draw one object for twenty minutes.
82. Combine two activities that have not been combined before.
83. Write about your day in an encyclopedic fashion. (i.e. organize by subject.)
84. Write a list of all the things you do to escape.
85. Cut a random shape out of several layers of a magazine. Make a collage out of the results.
86. Write an entry in code.
87. Make a painting using tools from the bathroom.
88. Work with a medium that is subtractive.
89. Write about or draw some of the doors in your life.
90. Make a postcard that has some kind of activity on it.
91. Divise a journal entry using "layers".
92. Divise an entry using "layers".
93. Write your own definition of one of the following concepts, sitting, waiting, sleeping (without using the actual word.)
94. List 10 of your habits.
95. Illustrate the concept of "simplicity".
For 96-100, write down and complete five of your own ideas!
Click here for a printable PDF file >>
12/06: Body tricks everyone should know!
| 1. If your throat tickles, scratch your ear! When you were 9, playing your armpit was a cool trick. Now, as an adult, you can still appreciate a good body-based feat, but you're more discriminating. Take that tickle in your throat; it's not worth gagging over. Here's a better way to scratch your itch: "When the nerves in the ear are stimulated, it creates a reflex in the throat that can cause a muscle spasm," says Scott Schaffer, M.D., president of an ear, nose, and throat specialty center in Gibbsboro, New Jersey. "This spasm relieves the tickle." 2. Experience supersonic hearing! If you're stuck chatting up a mumbler at a cocktail party, lean in with your right ear. It's better than your left at following the rapid rhythms of speech, according to researchers at the UCLA David Geffen School of Medicine. If, on the other hand, you're trying to identify that song playing softly in the elevator, turn your left ear toward the sound. The left ear is better at picking up music tones. 3. Overcome your most primal urge! Need to pee? No bathroom nearby? Fantasize about Jessica Simpson. Thinking about sex preoccupies your brain, so you won't feel as much discomfort, says Larry Lipshultz, M.D., chief of male reproductive medicine at the Baylor College of Medicine. For best results, try Simpson's "These Boots Are Made for Walking" video. 4. Feel no pain! German researchers have discovered that coughing during an injection can lessen the pain of the needle stick. According to Taras Usichenko, author of a study on the phenomenon, the trick causes a sudden, temporary rise in pressure in the chest and spinal canal, inhibiting the pain-conducting structures of the spinal cord. 5. Clear your stuffed nose! Forget Sudafed. An easier, quicker, and cheaper way to relieve sinus pressure is by alternately thrusting your tongue against the roof of your mouth, then pressing between your eyebrows with one finger. This causes the vomer bone, which runs through the nasal passages to the mouth, to rock back and forth, says Lisa DeStefano, D.O., an assistant professor at the Michigan State University college of osteopathic medicine. The motion loosens congestion; after 20 seconds, you'll feel your sinuses start to drain. 6. Fight fire without water! Worried those wings will repeat on you tonight? "Sleep on your left side," says Anthony A. Starpoli, M.D., a New York City gastroenterologist and assistant professor of medicine at New York Medical College. Studies have shown that patients who sleep on their left sides are less likely to suffer from acid reflux. The esophagus and stomach connect at an angle. When you sleep on your right, the stomach is higher than the esophagus, allowing food and stomach acid to slide up your throat. When you're on your left, the stomach is lower than the esophagus, so gravity's in your favor. 7. Cure your toothache without opening your mouth! Just rub ice on the back of your hand, on the V-shaped webbed area between your thumb and index finger. A Canadian study found that this technique reduces toothache pain by as much as 50 percent compared with using no ice. The nerve pathways at the base of that V stimulate an area of the brain that blocks pain signals from the face and hands. 8. Make burns disappear! When you accidentally singe your finger on the stove, clean the skin and apply light pressure with the finger pads of your unmarred hand. Ice will relieve your pain more quickly, Dr. DeStefano says, but since the natual method brings the burned skin back to a normal temperature, the skin is less likely to blister. 9. Stop the world from spinning! One too many drinks left you dizzy? Put your hand on something stable. The part of your ear responsible for balance -- the cupula -- floats in a fluid of the same density as blood. "As alcohol dilutes blood in the cupula, the cupula becomes less dense and rises," says Dr. Schaffer. This confuses your brain. The tactile input from a stable object gives the brain a second opinion, and you feel more in balance. Because the nerves in the hand are so sensitive, this works better than the conventional foot-on-the-floor wisdom. 10. Unstitch your side! If you're like most people, when you run, you exhale as your right foot hits the ground. This puts downward pressure on your liver (which lives on your right side), which then tugs at the diaphragm and creates a side stitch, according to The Doctors Book of Home Remedies for Men. The fix: Exhale as your left foot strikes the ground. 11. Stanch blood with a single finger! Pinching your nose and leaning back is a great way to stop a nosebleed -- if you don't mind choking on your own O positive. A more civil approach: Put some cotton on your upper gums -- just behind that small dent below your nose -- and press against it, hard. "Most bleeds come from the front of the septum, the cartilage wall that divides the nose," says Peter Desmarais, M.D., an ear, nose, and throat specialist at Entabeni Hospital, in Durban, South Africa. "Pressing here helps stop them." 12. Make your heart stand still! Trying to quell first-date jitters? Blow on your thumb. The vagus nerve, which governs heart rate, can be controlled through breathing, says Ben Abo, an emergency medical- services specialist at the University of Pittsburgh. It'll get your heart rate back to normal. 13. Thaw your brain! Too much Chipwich too fast will freeze the brains of lesser men. As for you, press your tongue flat against the roof of your mouth, covering as much as you can. "Since the nerves in the roof of your mouth get extremely cold, your body thinks your brain is freezing, too," says Abo. "In compensating, it overheats, causing an ice-cream headache." The more pressure you apply to the roof of your mouth, the faster your headache will subside. 14. Prevent near-sightedness! Poor distance vision is rarely caused by genetics, says Anne Barber, O.D., an optometrist in Tacoma, Washington. "It's usually caused by near-point stress." In other words, staring at your computer screen for too long. So flex your way to 20/20 vision. Every few hours during the day, close your eyes, tense your body, take a deep breath, and, after a few seconds, release your breath and muscles at the same time. Tightening and releasing muscles such as the biceps and glutes can trick involuntary muscles -- like the eyes -- into relaxing as well. 15. Wake the dead! If your hand falls asleep while you're driving or sitting in an odd position, rock your head from side to side. It'll painlessly banish your pins and needles in less than a minute, says Dr. DeStefano. A tingly hand or arm is often the result of compression in the bundle of nerves in your neck; loosening your neck muscles releases the pressure. Compressed nerves lower in the body govern the feet, so don't let your sleeping dogs lie. Stand up and walk around. 16. Impress your friends! Next time you're at a party, try this trick: Have a person hold one arm straight out to the side, palm down, and instruct him to maintain this position. Then place two fingers on his wrist and push down. He'll resist. Now have him put one foot on a surface that's a half inch higher (a few magazines) and repeat. This time his arm will cave like the French. By misaligning his hips, you've offset his spine, says Rachel Cosgrove, C.S.C.S., co-owner of Results Fitness, in Santa Clarita, California. Your brain senses that the spine is vulnerable, so it shuts down the body's ability to resist.
18. Read minds! Your own! "If you're giving a speech the next day, review it before falling asleep," says Candi Heimgartner, an instructor of biological sciences at the University of Idaho. Since most memory consolidation happens during sleep, anything you read right before bed is more likely to be encoded as long-term memory. Source: http://www.menshealth.com/cda/article.do?site= More Articles: Top ten reasons to get into Running |
12/06: 10 Traits that'll make a great girlfriend...
1. She's independentNo one wants a girlfriend they have to baby-sit. Once in a while, like if she's had a rough day at work, it's great to be her shoulder to cry on, but if she can't seem to function without you and is constantly after you, she will eventually make you feel like you're suffocating, which is a surefire way to get you running out the nearest exit. On the other hand, if she has her very own personality and opinions, can stand on her own two feet, both financially and emotionally, and is able to enjoy time away from you - while still missing you, of course - then she must be a great girlfriend.
2. She's intelligent
I hate to be the one to tell you this, but the bimbo routine gets real old, real fast. Instead of being the one in total control, you'll find yourself trying to figure out what she's really thinking behind those glazed eyes of hers - or if she's actually thinking at all. An intelligent woman will constantly surprise you and keep you on your toes. She won't let you get bored of her. Besides, it's nice to have something to talk about between all that chandelier-hanging sex.
3. She's sexual
While we're on the topic, a great girlfriend has to be sexually compatible with you. For instance, if you're into S&M and she's more the "fluffy lingerie" type, that's a problem. The two of you have to be on the same page - or, at least, she has to be willing to wear leather and use a whip from time to time. Of course, this doesn't imply that she has to know all the right moves straight away; it simply means that you and she have an undeniable attraction toward each other, and are able to communicate your desires verbally (or with physical cues). It is important that you please each other in the bedroom, or on top of the dryer - whatever the case may be.
4. She's beautiful
I know, this one is kind of obvious, but important nonetheless. A great girlfriend will not only want to look good for you, but also for herself. She should always look her best and be well put together - matching lingerie is a definite plus. You have to be proud to have her on your arm and enjoy the sight of her in any light. And this doesn't mean that she has to be a Heidi Klum clone. Remember that beauty is in the eye of the beholder, so if you think her full bottom or uncontrollable curls are beautiful, you're allowed.
5. She respects you
This is a biggie. Your woman must respect you. This means that she listens to you, even if she doesn't necessarily agree with what you're saying. And, of course, she never tries to demean or belittle you in any way, shape or form. A great girlfriend won't ever cause scenes in public or in front of your friends and family, and will always wait to discuss matters with you in private. If she respects you, chances are that she will behave in a tactful and diplomatic manner in most situations, which is definitely a good thing.
6. She lets you be a man
Do not - I repeat - do not get involved with a woman who tries to get you to eat cottage cheese and fruit for breakfast and insists that you give up poker night with the guys. You will end up resenting her more than you can imagine. A good girlfriend lets you be a guy in all your glory, poker night and all. If she's a great girlfriend, she'll even bring you and your buddies a couple of beers and make you some of her famous sandwiches. She has to understand that men and women are different and should allow you to be yourself. Just like you wouldn't deprive her of going shopping with her best girlfriend, she shouldn't expect you to give up the guys for her.
7. She's nagless
There is nothing worse than a nag! A great girlfriend knows this and chooses her battles wisely. She knows when to speak up and when to let it slide. You don't want a girlfriend who will give you hell for leaving a couple of dishes in the sink occasionally. However, if you live together and you stay out all night without calling her, and she lets you have it, then you're setting yourself up for disaster. This is a situation that nobody would let slide - not even a great girlfriend.
8. She gets along with friends and family
A great girlfriend will not only help your mom in the kitchen, listen to your dad's stories and hang out with your friends, but she will enjoy it. She'll make a real effort to get to know and love the most important people in your life. And she won't try to get you to ditch your best buds. She'll actually empathize with your brother's getting dumped and suggest that you guys take him out to cheer him up. Not only that, but your friends won't roll their eyes and moan when you mention that she'll be joining you guys when she gets off work (yes, women like this do exist).
9. She loves you
If you have found a woman who loves you for who you really are and not who you pretend or try to be sometimes, you should definitely hang on to her. A woman who doesn't try to change you is hard to find. Of course, all women have their slightly annoying habits that their mate has to contend with, but if she really loves you, she will be able to cope with these. Another way to know if she really loves you is by observing the way she looks at you and treats you on an everyday basis. If the sight of you doesn't seem to faze her either way, and she doesn't really seem to care about what you have to say, she's either playing very hard to get, or sees you as just some guy. But if a surprise visit or phone call from you makes her light up, there's no denying that she loves you.
10. She makes you want to be a better man
Stop making that face... any man who has a great girlfriend or wife will tell you that she makes him want to be a better man. She doesn't have to say or do anything; it just is that way. If you suddenly feel bad about how you treated your sister or find yourself trying to get your finances in order, you might want to think about your motivation for doing so. It could be love
07/06: More Streeview captures....
More street captures by the Google Van... check em out.

Entering an adult book store...

The Google team posing for the camera

"Taking photographs of students is prohibited," the sign reads...

A cat behind the window (the NYT identified the owner, Mary Kalin-Casey!)...

They just wanna play, not bite, I'm sure!![]()
05/06: 10 intereesting sights in Google Street View...
Drive around America's biggest cities in a black VW Beetle with a huge panoramic camera bolted to the roof, and you're sure to see something unusual along the way...

Google's magic van has captured some very interesting scenes as it journeyed through the streets. But in the days since the service was launched, numerous interesting sights have been spotted.
1. Someone apparently climbing over a fence in San Francisco
2. Borat peeking out of a window in San Jose, California
3. The already infamous 'Hot Babes' poster van driving alongside the Google truck in Las Vegas
4. A girl bending over, and two guys watching her...
5. Ambulance driver stops for a sandwich
7. Strange, headless figures next to what looks like a newly dug grave